PARTNER II SAPIEN 3 Trial – TAVR Outperforms SAVR – Again!
PARTNER II SAPIEN 3 Trial – TAVR Outperforms SAVR – Again!
The one year results of the PARTNER II,...
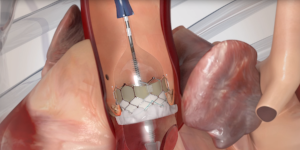
Is TAVR1 suitable for everyone? As originally approved, transcatheter aortic valve replacement was intended only for high risk patients who could not otherwise endure an open-surgical (or sAVR) procedure; the selection criteria was strict. As reported at the American College of Cardiology’s 65th Scientific Session:
“over 40,000 cases of TAVR (were) performed in the first four years after the technology was approved by the FDA,” said John D. Carroll, M.D., professor of medicine and director of interventional cardiology at the University of Colorado Hospital and lead author of the study2.
With time, the publication of positive long-term surgical outcomes and the fine-tuning of TAVR design (e.g., third generation valves), the inevitable “indication creep” began to occur. For many surgeons, TAVR was deemed to be a better solution – clinically and financially -- for severe aortic stenosis for a broader range of patients, not just those at highest risk. First to benefit were intermediate risk patients, and now, TAVR for low-risk patients is the inevitable target of research and development aiming at formal approval. In the near-term future, TAVR may truly be deemed suitable for everyone.
First, let’s review the facts about TAVR:
The original PARTNER trial studying high-risk patients was completed in 2010, and CMS issued its National Coverage Determination in November 2011. The one-year results of the PARTNER 2 TAVR trial were announced in April 2016, and proved that TAVR was a reasonable alternative to sAVR in “intermediate” risk patients, estimated at 40% to 50% of total surgical volume. There was already considerable indication creep at play with this group of patients, as they were often receiving the procedure on an “off-label” basis. Of note is the fact that intermediate risk patients already were “officially” receiving TAVR in Europe.
On July 1, 2016, CMS granted the MedStar Research Institute an investigational device exemption to conduct a prospective TAVR trial to assess the safety and feasibility of TAVR in patients who are at least 65 years old, have severe aortic stenosis and are at low risk for surgical aortic valve replacement (sAVR). The study will take place at MedStar Washington Hospital Center in Washington, D.C., under Dr. Ron Waksman, the principal investigator. They will complete the study by January 2021 by comparing 200 patients who prospectively undergo transfemoral TAVR with a control group of patients who had undergone bioprosthetic sAVR at the same site for the previous 36 months.
Note that the targeted patient populations for this study are patients at “low risk.” Assuming that the results of this study are positive (and there is every expectation that they will be), then TAVR appropriateness criteria will be significantly broadened to include not only intermediate and high-risk patients, but TAVR for low-risk patients as well, accessing the 1.5 million aortic stenosis market.
For hospitals that were early adapters and could meet the strict CMS guidelines for Medicare coverage, their investment should pay off as the market will continue to expand through intermediate risk patients and inevitably into the low-risk market. These hospitals should be positioned to reap the reward of early market entry, through continued market share development, geographic penetration and brand enhancement.
Hospitals seeking to enter the market for the first time must first face the hurdle of CMS participation requirements, which place a strong experiential burden on the entire heart team3. Second, new entrants will face the potential disadvantage of having established competition in their marketplace. That competition will continue to build volume and market share as the
appropriateness indicators are broadened and the market grows. These factors alone may deter any new entrant into this market.
The countervailing argument is the fact that this market is growing, reimbursement is reasonably strong and probably will be applicable to an ever larger target population. Any growing market is a reasonable potential target for program development and investment. Beyond the self-assessment question of “can it be done,” comes the equally important question of “should it be done.” With the latter question, each hospital will need to address issues of:
Given its use with high risk patients, its increasing use in intermediate risk patients, and its potential use in low risk patients, can TAVR for everyone become a reality over the next 5 years or so? For those hospitals not yet performing TAVR, it is prudent to incorporate this issue into their mid and longer range strategic planning.
If you are interested in learning more about developing TAVR programs for adults with aortic stenosis, please contact CFA at (949) 443-4005 or by email at CFA@charlesfrancassociates.com.
Footnotes
1 TAVR enables the placement of a stent-based tissue aortic heart valve into the body via a catheter inserted through an incision in the groin and threaded up into the heart through the arteries. Once placed, the valve begins to function immediately and symptoms are alleviated. An alternative placement can be made transapically, through the heart wall rather than transarterially. It is performed in an OR or a hybrid cath lab.
2 When the FDA approved the first transcatheter aortic heart valve in November 2011, the national Transcatheter Valve Therapy (TVT) Registry was created to track patient outcomes from the use of the device. The registry, which is jointly maintained by the American College of Cardiology and the Society of Thoracic Surgeons, now contains data on over 50,000 patients whose TAVR procedures were performed at nearly 400 hospitals in the United States. The database is designed to provide information that can help hospitals improve the quality of care for patients with severe aortic stenosis and help patients make informed decisions about this new form of heart valve replacement. Dr. Carroll serves on the Steering Committee of the TVT Registry.
3 Inclusive of individual minimum sAVR volumes per heart surgeon and interventional cardiologist (including balloon aortic valvuloplasty), plus specifications for additional members of the team such as echocardiographers, imaging specialists, heart failure specialists, cardiac anesthesiologists, intensivists, nurses, social workers and others.
PARTNER II SAPIEN 3 Trial – TAVR Outperforms SAVR – Again!
The one year results of the PARTNER II,...
CMS announced its long-awaited updated transcatheter aortic valve replacement (TAVR) national...