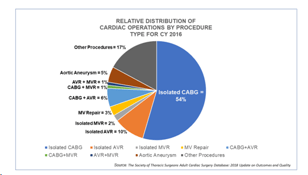
CMS announced its long-awaited updated transcatheter aortic valve replacement (TAVR) national coverage determination (NCD) proposal on March 26, 2019.
Based upon the proposed changes from its original May 2012 coverage determination, the updates, if formally adapted, could be a gift to low-volume cardiac surgery programs – and lower-volume cardiology programs in general – nationwide! The pressure was on CMS to increase access to and availability of TAVR for more hospitals from the hospital industry by lowering the existing volume requirements. The counter was the professional societies that believed keeping, or even strengthening the current requirements would help insure a quality product (the classic volume = quality issue). It appears the mounting years of TAVR study data highlighting safety and efficacy has strengthened the hospital industry’s case and helped it win the debate over balancing access and outcomes!
The proposed CMS decision memo outlines specific hospital infrastructure requirements, such as needing on-site heart valve surgery and interventional cardiology programs, along with a post-procedural intensive care unit experienced in managing patients following open-heart valve procedures.
The heart of the draft determination are the proposed volume requirements, which requires the following for hospitals to begin a program and receive reimbursement for the procedures:
- At least 50 open-heart surgeries in the year prior to starting a TAVR program.
- At least 20 aortic valve-related procedures in the two years before program initiation.
- Under the current NDR, 25 AVRs in one year, or 50 AVRs in two years prior to new service.
- At least 300 percutaneous coronary interventions (PCIs) per year.
- Currently, greater than 400 PCIs per year. Current requirement for 1,000 catheterizations per year has been deleted.
- At least two cardiac surgeons, including one with at least 100 career open-heart surgeries and 25 aortic valve surgeries.
- An interventional cardiologist with at least 100 career structural heart procedures or at least 30 left-sided structural procedures annually, along with device-specific training from valve manufacturers.
- Currently, surgeon also had to have 30 left-sided structural procedures of which 60% had to have been balloon aortic valvuloplasty.
In order to maintain reimbursement for a TAVR program, the proposal requires centers to have:
- At least 50 AVRs (TAVR or SAVR) annually or 100 every two years, including 20 or 40 TAVRs over those respective timeframes.
- Currently, 25 TAVR/SAVRs in one year, or 50 in two years
- 300 or more PCIs per year.
- At least one interventional cardiologist and two cardiovascular surgeons on staff.
Another proposed change from the current national coverage determination (NCD), is that CMS would require just one surgeon to sign off on the multidisciplinary evaluation for TAVR, SAVR or palliative care. The current NCD requires a two-surgeon signoff. Again, a gift to smaller volume programs.
The 30-day public comment period on the proposal is now open and CMS plans to make a final decision on the NCD within 60 days of the comment period ending.
Low-volume cardiac surgery programs could benefit from the decreases in required volumes for combined SAVR, overall catheterization procedures, PCIs and going-forward TAVRs under the proposed NCD. If adapted, these changes will significantly impact entry into this new market. CFA has several client hospitals that are following these developments closely, on the cusp of making a decision to move forward – unable to meet the existing requirements, but probably able to meet the proposed changes. We have written extensively about the TAVR question – whether or not to proceed with TAVR under the best of circumstances.
Structural heart services[i] including TAVR (and also transcatheter mitral valve repair/replacement) are complex and demanding services. Beyond the question of “can we meet the current and/or proposed standards,” comes the larger, more complex question of “should we enter this market?” With the latter question, each hospital will have to assess its own corporate strategy, internal existing/potential volumes, referral patterns and practices, payer mix/procedural cost/reimbursement, capital investment, operational capabilities, competition within the marketplace, physician leadership and capabilities, and other pertinent questions.
If you are interested in learning more about low-volume cardiac surgery programs strategies, please download our updated and expanded white paper (see Low-Volume Cardiac Surgery Programs: Grow, Consolidate or Divest: Self-Preservation Strategies and Excellence Expectations). If you are interested in cardiac services strategic development, service expansion and/or other programmatic needs for cardiovascular or other services, please contact CFA at (949) 443-4005 or by e-mail at cfa@charlesfrancassociates.com.

[i] See also Valve Surgery Trends and Implications, posted June 28, 2018, and Mitral Valve Surgery Trends and Implications, posted March 14, 2019.